By Marius Zega, Keshe Romanian Association; from the December edition of the Plasma Times
Table of Contents
Overview
In the “Universal Order of Creation of Matter”, M.Keshe describes that everything has been created out of the primordial neutron, that decayed into a proton and an electron; in this process residual plasma fields are released that can manifest as light or energy.
- Hydrogen
(H)
Is created out of the proton and electron that came out of the decayed neutron
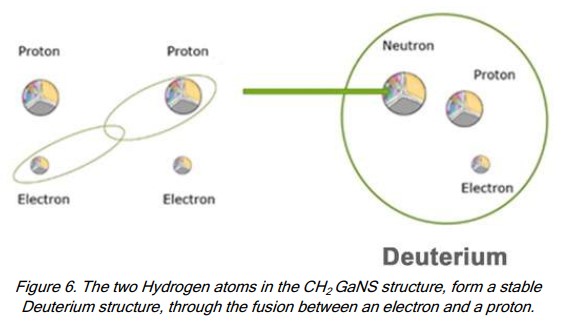
Atomic mass=1. - Deuterium
(H2)
Is created when 1 neutron is added to H
Atomic mass=2;
Fuel of the future
It can be magnetic or gravitational
GaNS-H2 particles interact with magnets
The colour is black or yellow - Tritium
(H3)
Is created when 1 neutron is added to H2
Atomic mass=3
H3 has a high magnetic field strength
It emits no radioactivity in it’s plasmatic state.
The Neutron Plasma contains all the plasmatic fields; it is therefore essential for all and any applications, be it energy, creation of materials, transport etc.
Producing GaNS-H3
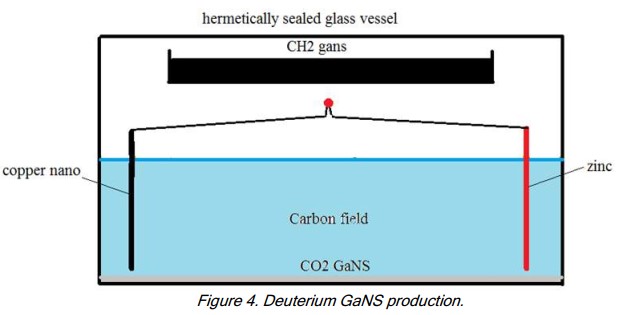
- Use a standard CO2 production unit inside a glass container with a hermetic lid: Cu-np + Zn + LED in 3% salt water solution. The Cu-np and Zn are placed 12-14cm apart.
- Install another, uncovered glass vessel at least 4cm above water level and above the plates and fill it with GaNS-CH3.
- Close the lid of the glass container and let it stand for 10-12 days. A yellow or yellow-blue liquid will form in the CH3 vessel; this is the GaNS-H3.
- Store the GaNS-H3 in a hermetically closed glass container with a glass lid and a rubber gasket (jar to store pickles).
- You can speed up the process by applying a small DC current on the 2 plates.
In the process the Cu-np with the Zn plate create the field of C (carbon). The C cannot be taken from the atmosphere because the container is closed. So the only place from where the C can come is from the vessel with the CH3; leaving only H3 in the vessel.
Alternative method
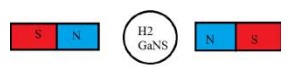
If you place GaNS-H2 in between two North poles of two magnets, the magnetic field gets enhanced, so it gains a Neutron and becomes Tritium.
Producing GaNS-H2
1. Method
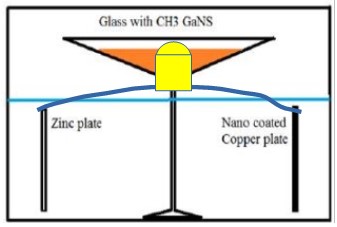
- Use a standard CH3 production unit inside a glass container with a hermetic lid: Cu-np + Fe-galvanized in 3% salt water solution. The Cu-np and Fe-galvanized are placed 12-14cm apart.
- Install another, uncovered glass vessel at least 4cm above water level and above the plates and fill it with GaNS-CH3.
By this method the field strengths of C (carbon) and H (hydrogen) are generated which extract 1x C and 1x H from the open CH3 GaNS container, leaving only H2
2. Method
-
Use
a CH3 GaNS production kit and connect the metal plates to a power
supply: + to the Cu-np plate and – to the Fe-galvanized plate;
<1.5VDC/50mA
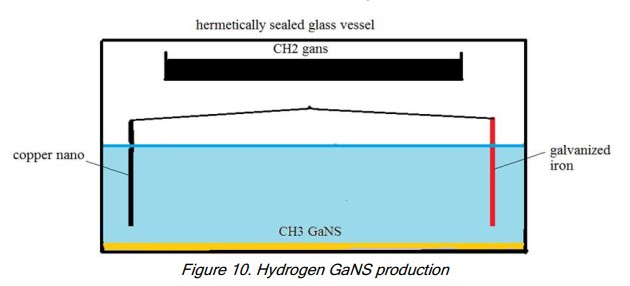
We obtain GaNS-CH2 of black colour - We will obtain H2 by extracting the C (carbon) from the CH2: Use a standard CO2 production unit inside a glass container with a hermetic lid: Cu-np + Zn + LED in 3% salt water solution. The Cu-np and Zn are placed 12-14cm apart.
- Install another, uncovered glass vessel at least 4cm above water level and above the plates and fill it with GaNS-CH2.
3. Method
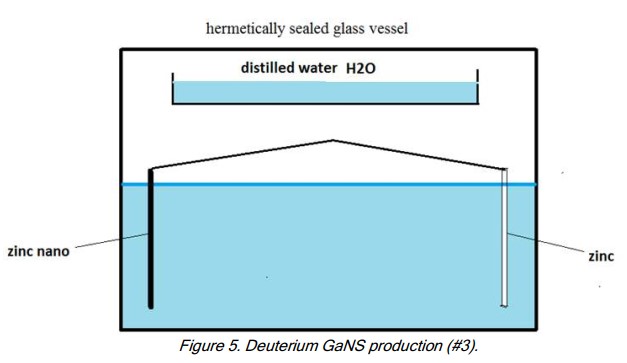
- Use a standard ZnO production unit inside a glass container with a hermetic lid: Zn-np plate + Zn plate in 3% salt water solution. The Zn-np and Zn are placed 12-14cm apart.
- Install another, uncovered glass vessel at least 4cm above water level and above the plates and fill it with distilled water.
After the oxygen of the sealed air quantity is exhausted the oxygen from the distilled water (in plasma state, behaving like GaNS-H2O), leaving H2 in the floating container. The water will turn black.
This method can also be used to reduce CO2 to GaNS-C.
4. Method
This method uses 3 dynamic reactors with balls filled with GaNS-H3 in different proportions. In one reactor GaNS-H3 will remain, the second will produce GaNS-H and the third GaNS-H2.
Deuterium GaNS need to be stored in a hermetically sealed container, because it reacts strongly with anything around it.
5.Method
Place GaNS-H3 between between the north pole of one magnet and the south pole of another magnet; or – alternatively between two South poles of two magnets. The Tritium GaNS loses a Hydrogen atom and is reduced to Deuterium GaNS.

Producing GaNS-H
- Place GaNS-CH2 in an open vessel over a hermetically sealed CH3 production setup. Here the CH3 setup pulls the plasma field of C from the CH2 leaving the GaNS-H; it has a blue color.
Producing GaNS-Neutron
1. Method
Place
some GaNS-H2 between the north pole of one magnet and the south pole
of another magnet; or – alternatively between two South pole of two
magnets.
The magnetic interaction extracts the magnetic field
from the GaNS-H2 which loses one H atom and is thus reduced to a
Neutron.

The difference between Hydrogen and Deuterium as well as the difference between Deuterium and Tritium is just a Neutron. That is why the GaNS-Neutron can be obtained from the interaction of both the pairs. However, the GaNS-Neutron obtained from H/H2 differs from the one you get from H2/H3.
2. Method
For the production you set up 3 double core glass ball reactors on motors: the external reactor is filled with GaNS-H3 in distilled water salt solution; the middle ball is filled with distilled water salt solution; and the inner core is filled with GaNS-H2 in distilled water salt solution. Neutron plasma gets captured in the distilled water salt solution of the middle ball. Salty water is the best Neutron capture medium because it slows down the plasma fields to their matter state.
It important to know that GaNS-Neutron is very reactive and decays easily into a proton and an electron of an element which is determined by the plasmatic strength of the environment – especially our sou. That means the GaNS-Neutron is an ideal agent to produce any material.