Table of Contents
The production of the 4 basic GaNS
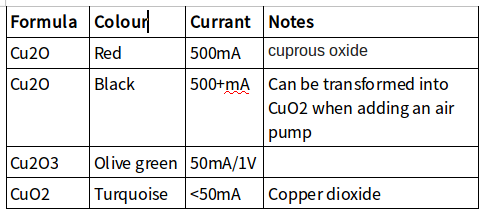
GaNS-CO2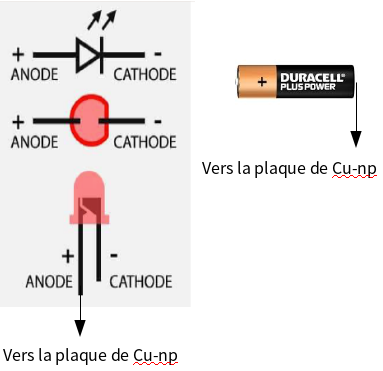
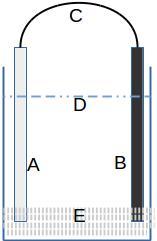 1. In a plastic container install a nano-coated copper plate (Cu-np) (B)
1. In a plastic container install a nano-coated copper plate (Cu-np) (B)
2. Install another Zinc electrode (A)
3. Install a wire with a green LED between the two plates; the (+) terminal of the LED is a little longer – it is connected to the Cu-np plate and the other terminal to the Zn plate
4. Dissolve salt in demineralized or distilled water at a concentration of 3.5% to 10% (D)
5. After a certain time, the GaNS will settle at the bottom of the container (E)
You can use a low source of electricity to accelerate the production of GaNS. The positive pole of the battery is connected to the Zn plate, the negative pole to the Cu-np plate.
GaNS-ZnO
This GaNS is produced using the same method as CO2 – only there is no LED between the two plates but a simple wire.
According to this method, pure ZnO is not produced but 95% GaNS-ZnO and 5% GaNS-CO2. To produce pure GaNS-ZnO, the copper-nano plated plate (Cu-np) is replaced by a zinc nano plated plate (Zn-np). The decisive metal remains the simple zinc plate. The two plates are simply connected by a wire. This installation provides 100% ZnO.
GaNS-CH3
 To produce GaNS-CH3, you use a Cu-np plate; and the decisive metal plate is galvanized iron (chicken mesh); all in a saline solution.
To produce GaNS-CH3, you use a Cu-np plate; and the decisive metal plate is galvanized iron (chicken mesh); all in a saline solution.
The plate of the decisive metal is galvanized iron; a metal composed of zinc (Zn, the galvanizing layer) and iron (Fe). In this arrangement, C (Zn and Cu-np) and then H (Fe and Cu-np) are produced.
Here is the calculation that determines what we get:
Zn(65) – Cu-np (59) = 6 = 1 carbon (C)
Fe (56) – Cu-np (59) = 3 = 3*1 hydrogen (H)
Result: 1 carbon and 3 hydrogen = CH3
Zn has an atomic mass of 65, Fe one of 56 and Cu-np of 59. The difference between Cu-np and Fe = 59-56 = 3 (3 hydrogens); the difference between Zn and Cu-np = 65-59 = 6 (Carbon). Both sets give us: Carbon + 3x Hydrogen = 6+3=9 = CH3.
CH3 is the essential sugar of this planet. The amino acids that are formed on its surface are linked to Fe, and therefore to the haemoglobin in our blood. Amino acids also have a connection with the atmosphere of our planet.
GaNS-CuO
CuO is produced by a Cu-np plate and the decisive metal is a simple Cu plate. The two plates are linked by a wire. If you add an LED, the GaNS produced will have larger and more spaced particles; however, production will be slower.
The process can provide different copper oxides, depending on the installation and whether you use electricity: