All the chemical elements of our planet are organized in the periodic table of elements; we find for each element its characteristics. On the periodic table we find the following information for the element Copper:
 its abbreviation (Co for Copper)
its abbreviation (Co for Copper)- its atomic number (29)
- its atomic mass (63,546)
- the number of protons, neutrons and electrons
On the following table you see the characteristics of the elements that are used to make the 4 basic GaNS.
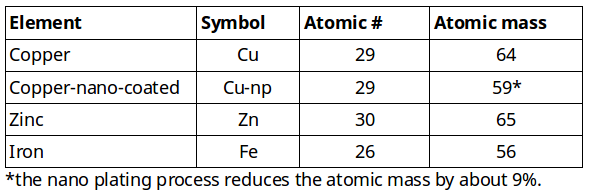
The sum of protons and neutrons corresponds to the atomic mass: 29+35=64. The number of neutrons is calculated by subtracting the number of protons from the atomic weight: 64 (atomic weight) – 29 protons = 35 neutrons.
The characteristics of the periodic table of elements describe the elements at the material level; there is not yet a periodic plasma table of elements, but conclusions can already be drawn about the plasma force of each element. The atomic mass of each element can be taken as an indicator of its plasma strength – the heavier an element, the stronger its plasma field.
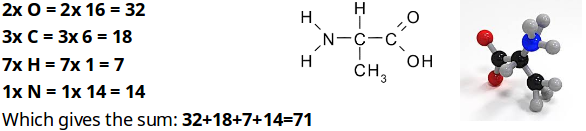
To find the field strength of a molecule or substance, the atomic weights of all the elements of which the substance is composed are added together. For example, the amino acid ‘Alanine’ contains: